IMPETIGO
Luciano Schiazza M.D.
Dermatologist
c/o InMedica - Centro Medico Polispecialistico
Largo XII Ottobre 62
cell 335.655.97.70 - office 010 5701818
www.lucianoschiazza.it
Impetigo is a highly contagious, gram-positive bacterial skin infection of the superficial layers of the epidermis. It occurs most commonly in children, with a peak incidence between ages 2 and 6, but can also affect adults, especially when people live in a confined environment. Adults often develop impetigo from close contact with infected children.
The name derives from the latin “impetere” (to assail).
Impetigo is contagious, primarily from direct contact with someone who has it as by closed contact in school, nurseries, day-care centers, nurseries, grade schools, between household members, classmates or teammates. Participation in contact sports, crowded living conditions, poor personal hygiene, or an unhygienic work environment encourages contamination.
But sometimes also towels, toys, clothing, or household items can be way of infection. Impetigo is most likely to occur in warm and humid environments and climates. Clusters in families and mini epidemics in institutions and day-care centers are occasionally reported. The presence of eczema predispose a person to developing impetigo. Impetigo can affect people of all races, with equal incidence in males and that in females, and of all ages but is most common in children 2-5 years of age.
Common bacteria causing impetigo are Staphylococcus(from the greek word staphylè: bunch of grapes) Aureus (because of the yellow-gold color in culture) and group A beta hemolytic streptococci (GABHS also known as Streptococcus pyogenes). Intact skin is usually resistant to colonization or infection by S. aureus or GABHS, because these bacteria require an epithelial cell receptor (fibronectin) for colonization, unavaible on intact skin. Disrupted surfaces (such as that comes from cuts and scrapes) may reveal fibronectin receptors and allow for clonization. Also factors such as high temperature or humidity, preexisting cutaneous disease, young age, or recent antibiotic treatment, modifying the usual skin flora can facilitate transient colonization by GABHS and S aureus.
Common causes of a break in the skin:
-
Scratching
-
Dermatophytosis
-
Varicella
-
Herpes simplex
-
Scabies
-
Pediculosis
-
Thermal burns
-
Surgery
-
Trauma (cut)
-
Radiation therapy
-
Insect bites
A common toddler impetigo experience is the development of impetigo at the nasal openings inflamed by the prominent nasal drainage associated with a cold. In this situation, skin integrity is often disrupted by the continuous covering of purulent nasal discharge. Approximately 30% of the population is colonized in the anterior nares by S aureus. Some individuals colonized by S aureus experience recurrent episodes of impetigo on the nose and lip. Bacteria can spread from the nose to healthy skin within 7-14 days, with impetigo lesions appearing 7-14 days later.
Recurrent impetigo infections may be associated with staph or strep bacteria residing in the nose and spreading from to other parts of the skin. Nasal carriage should be determined using cultures of the nares of all individuals within a household.
Immunosuppression by medications (eg, systemic corticosteroids, oral retinoids, chemotherapy), systemic diseases (eg, HIV infection, diabetes mellitus), intravenous drug abuse, and hemodialysis encourages bacterial growth.
After initial infection, new lesions may be seen in areas with no apparent break in the skin. Frequently, however, upon close examination, these lesions will demonstrate some underlying physical damage. GABHS can be detected in the nose and throat of some individuals 2-3 weeks after lesions develop, although they do not have symptoms of streptococcal pharyngitis. This is because impetigo and pharyngitis are caused by different strains of the bacteria.
Impetigo is usually due to pattern D strains, whereas pharyngitis is due to pattern A, B, and C strains. Approximately 10% of individuals are colonized with S aureus in the perineum and, more uncommonly, in the axillae, pharynx, and hands. Individuals who are permanent carriers serve as reservoirs of the infection for other people. Most healthy persons transiently harbor S aureus as part of their microbial flora.
S aureus often passes from one individual to another through direct hand contact. Individuals with impetigo frequently recall exposure to a person who is a known carrier of S aureus or streptococcal organisms, has a pyoderma, or has a skin condition (eg, atopic dermatitis) that predisposes that individual to be an S aureus or streptococcal carrier. If an individual is in close contact with others (eg, household members, classmates, teammates) who have GABHS skin infection or who are carriers of the organism, the normal skin of that individual may be colonized. Once the healthy skin is colonized, minor trauma, such as abrasions or insect bites, may result in the development of impetigo lesions within 1-2 weeks. An important diagnostic clue is the clustering of lesions around facial orifices and/or exposed areas of the body. There are two types of impetigo:
-
bullous impetigo, which causes large, painless, fluid-filled blisters
-
non-bullous impetigo, which is more contagious than bullous impetigo and causes sores that quickly rupture (burst) to leave yellow-brown crust.
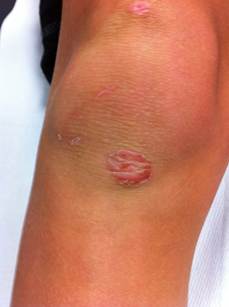
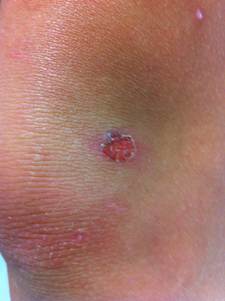
Both may lack signs of acute infection (e.g. dolor, calor, tumor), masquerading themselves as noninfectious disease.
Bullous impetigo It is caused exsclusively by staphylococcus aureus bacteria (Coagulase-positive group II S aureus, most often phage type 71, is the predominant causative organism).
These bacteria produce a toxin, termed exfoliatins A, B and D, that loss cell-to-cell adhesion in the granular cell layer of the epidermis (the top skin layer) causing the formation of blisters (the medical term for blister is bulla) and skin sloghing. One of the target proteins for exotoxin A is desmoglein I, which maintains cell adhesion. These molecules are also superantigens that act locally and activate T lymphocytes.
Usually lesions consist of thin-roofed, flaccid, superficial, delicate, transparent, bullae. These are less than 3 cm in diameter and contain a clear yellow-colored fluid. Often, these quickly appear, but it is unusual to see intact bullae because they are very fragile and spontaneously break and drain. Then lesions desiccate and a dark crust or scalded skin appearance with a collarette of scale at the perifery commonly develops during the final stages of development. With healing, this crust will resolve.
There is minimal or no surrounding erythema and no regional lymphadenopathy. Sometimes lesions appear circinate because of central healing. Lesions typically appear on intact skin in various skin areas but may secondarily invade preexisting lesions (eg, eczema) to cause generalized lesions. Bullous impetigo affects most frequently neonates but also can occur in older children and adults.
In infants, extensive lesions may be associated with systemic symptoms such as fever, malaise, generalized weakness, and diarrhea (usually absent in impetigo contagiosa). Rarely, infants may present with signs of pneumonia, septic arthritis, or osteomyelitis. Bullous impetigo is less contagious than nonbullous impetigo.
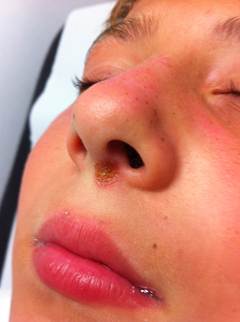
Non-bullous impetigo: also known as impetigo contagiosa, is the more common form, constituting approximastely 70% of impetigo cases. It accounts for approximately 10% of all cutaneous problems in pediatric clinics. It is more contagious than the bullous type. It is caused by both Staphylococcus aureus and group A beta hemolytic streptococci (GABHS also known as Streptococcus pyogenes). Most infections begin as a streptococcal infection, but staphylococci replace the streptococci over time.
This form initially presents as small red papules similar to insect bites that rapidly evolve to tiny vesicles or pustules that rupture and scab over with a characteristic honey-colored crust (due to serous content dried) over the erosion, usually measuring less tha 2 cm. After treatment lesions heal without scarring. This entire process takes about one week.
Skin on any part of the body can be involved, but lesions are commonly located on the face (around the mouth and the nose) and extremities (exposed parts of the body such as arms, legs) or in areas with a break in the natural skin defense barrier. The palms and soles are spared. Rapid spread follows by contiguous extension or to distal areas through inoculation of other wounds from scratching. Little or no surrounding erythema or edema is present.
There may be swollen but non-tender lymph nodes (glands) nearby (regional adenopathy). Multiple lesions generally occur at the same site, often coalescing. The affected area of skin may enlarge as the infection spreads peripherally. Pruritus of infected areas may result in excoriations due to scratching. Patients with impetigo may report a history of minor trauma, insect bites, scabies, herpes simplex, varicella, or eczema at the site of infection. Any history of preexisting skin disease should raise the clinician's index of suspicion. Common impetigo is the term applied when the infection occurs in preexisting wounds. Impetigo as a secondary infection of preexisting skin disease or traumatized skin has also been referred to as impetiginous dermatitis.
Methicillin-resistant S aureus (MRSA) which can be hospital or community acquired, is an increasing common cause of impetigo, more often in the nonbullous form than the bullous form. MRSA has been isolated in as many as 20% of bullous impetigo cases.
Among the risk factors for hospital-acquired MRSA are the following:
-
Working in a health care center
-
Hospitalization within the past year
-
Residence in a long-term facility
-
Having a chronic indwelling catheter or medical device
Community-acquired MRSA is a growing problem. Community-acquired MRSA is seen in greater frequency in closed populations in prisons, day care centers, and athletic teams, as well as in patients with diabetes or an underlying skin condition. The prevalence in these communities has been reported to be as high as 50%.
Diagnosing impetigo is generally straightforward and based on the clinical appearance. Occasionally, other conditions may look something like impetigo. It is important to note that not every blister means an impetigo infection. Infections such as
-
tinea ("ringworm")
-
scabies (mites).
At times, other infected and noninfected skin diseases produce blister-like skin inflammation. Such conditions include:
-
herpes cold sores
-
npox
-
poison ivy
-
skin allergies
-
eczema
-
insect bites
Secondary infection of these other skin lesions may sometimes occur.
Because of the contagious nature, children should not return to daycare or school until 24 hours after the initiation of appropriate antimicrobial therapy. Caretakers should be instructed about hygienic issues and prevention. A history of poor hygiene and crowded living situations are common. This infection is transmitted by direct contact and by fomites, including hygiene items, clothing, and toys.
To minimise the risk of impetigo spreading, it's also advisable to:
-
avoid touching the sores
-
wash hands regularly
-
not share flannels, sheets or towels
-
keep children off nursery, playgroup or school until their sores have dried up
Beyond the neonatal period, patients who receive early and appropriate therapy have an excellent chance of recovery without complications.
Complications Acute poststreptococcal glomerulonephritis (APSGN) is a rare complication of nonbullous impetigo from nephritogenic strains of GABHS. The onset of poststreptococcal glomerulonephritis appears 18-21 days after infection.
No difference between the clinical appearance of impetigo due to nephritogenic and impetigo due to nonnephritogenic strains has been observed. It occurs usually 10 days after impetigo lesions first appear, but it can occur from 1-5 weeks later, with pedal edema and hypertension. Most often, these are children aged 2-7 years. Transient proteinuria and hematuria may occur during impetigo and resolve before renal involvement develops. The frequency of APSGN varies widely, depending on the strain of GABHS.
Many GABHS strains have no nephritogenic potential, but types M-60 and M-49 cause APSGN in 70% and 25% of cases, respectively. Antibiotic treatment does not prevent the development of APSGN, most likely because activation of the immune response precedes antibiotic treatment, but it limits the spread of the disease to other individuals. Interestingly, in certain tropical and subtropical climates, skin infection is the most common predecessor of APSGN. Uncommon complications include the following:
-
Scarlet fever
-
Osteomyelitis
-
Septic arthritis
-
Septicemia
-
Guttate psoriasis
-
Cellulitis
-
Erysipelas
-
Erythema multiforme
-
Urticaria
-
bacterial endocarditis
Neonates have a much higher incidence of developing a more generalized infection and meningitis. Cellulitis, lymphangitis, and suppurative lymphadenitis may occur in as many as 10% of patients with impetigo. No correlation between the amount of impetiginous lesions and the involvement of the surrounding soft tissues, lymphatics, or regional lymph nodes has been observed. Cellulitis rarely follows bullous impetigo.
If the exfoliative toxins are absorbed into the bloodstream, staphylococcal scalded skin syndrome can result. This occurs more commonly in younger children, who have not developed antibodies against this toxin. Rheumatic fever, however, has never been reported following streptococcal impetigo; rather, it develops after streptococcal pharyngitis.
Differential diagnosis
Alternative diagnostic possibilities are key to consider in recurrent cases or in those that do not respond quickly to treatment. Tinea is a very common cause of impetigo-like infection of the skin. Herpetic impetigo also is a very common and often missed mimic. Atopic patients, in particular those with extensive eczema, are a high-risk group. In these patients viral culture is recommended. Pemphigus vulgaris, rare in children.
Pemphigus foliaceus normally presents in adults. Clinically and histologically can mimic impetigo. Folliculitis, a superficial hair follicle infection. If this diagnosis appears possible, it should also consider pseudomonas folliculitis. Follicular mucinosis can be especially similar to impetigo in clinical presentation, although often without crust. Erysipelas is a skin infection typically caused by group A beta-hemolytic streptococci, as are many cases of nonbullous impetigo.
However, erysipelas manifests as a sharply demarcated erythematous plaque caused by dermal inflammation. Insect bites may resemble impetigo or may be a site for its development, emphasizing the need for a detailed history. Impetigo in an atypical location, such as the scalp, should warrant investigation for head lice. Other problems to be considered in the differential diagnosis of nonbullous impetigo include the following:
-
Cutaneous candidiasis
-
Kerion
-
Inflammatory dermatophytosis
-
Dermatophytic infections
-
Discoid lupus erythematosus
-
Sweet syndrome (acute febrile neutrophilic dermatosis)
Other problems to be considered in the differential diagnosis of bullous impetigo include the following:
-
Linear immunoglobulin A bullous dermatosis
-
Bullous pemphigoid reactions
-
Bullous lupus erythematosus
-
Bullous scabies
-
Dermatitis herpetiformis
-
Bullous-fixed drug reaction
Treatment
Impetigo is not serious and is very easy to treat. Topical antibiotics, systemic antibiotics, or a combination of both is effective therapy for impetigo. Empiric bacterial coverage is aimed at eradicating Staphylococcus aureus and group A beta-hemolytic streptococci (GABHS; also known as Streptococcus pyogenes).
Mild impetigo can be handled by gentle cleansing, removing crusts, and applying topical antibiotics. More severe or widespread impetigo, especially of bullous impetigo, may require oral antibiotic medication.
In recent years, more staph germs have developed resistance to standard antibiotics. Bacterial culture tests can help guide the use of proper oral therapy if needed. If clinical suspicion supported by culture results show other bacteria, such as drug-resistant staph (methicillin-resistant Staphylococcus aureus or MRSA), other antibiotics may be necessary, guided by laboratory results (culture and sensitivity tests).
Treatment produces a higher cure rate (lesions usually resolve after 7-10 days) and reduces the spread of infection to other parts of the body (via inoculation) or to other people. Scarring is unusual, but postinflammatory hyperpigmentation or hypopigmentation may occur. If lesions persist beyond after a week of treatment, cultures should be performed to look for resistant organisms. Antihistamines may be prescribed for symptomatic relief in patients with pruritus.
Topical antibiotic therapy Topical antibiotic therapy is considered the treatment of choice for individuals with uncomplicated localized impetigo because eradicates isolated disease and limits the individual-to-individual spread. It is not indicated for extensive lesions. Topical agent is applied after removal of the infected crusts and debris with soap and water. Disadvantage of topical treatment is that applying topical medications to extensive lesions is difficult. The advantages of topical antibiotics include the following:
-
Low risk of systemic adverse events
-
Higher concentration of the antibiotic when applied to the affected area
-
Smaller amount of drug is used
-
Lack of effect on intestinal florae
-
Low cost
-
Ease of administration to a young child
The disadvantages of topical antibiotics include the following:
-
Potential production of irritant and allergic contact dermatitis
-
Decreased penetration in the affected area
-
Potential rapid appearance of bacterial resistance
-
Potential alteration of cutaneous flora
-
Potential systemic absorption and consequent toxic effects
Options include:
-
Mupirocin 2% ointment
Mupirocin is a naturally occurring antibiotic produced by fermentation of Pseudomonas fluorescens. The mechanism of action of mupirocin is via inhibition of bacterial protein synthesis. Mupirocin ointment (Bactroban) has been used for both the lesions and to clear chronic nasal carriers. It is equally effective as oral cephalexin. Unfortunately, S aureus and MRSA resistance to mupirocin has emerged at estimated rates ranging from 5-10%.
-
Retapamulin
Retapamulin is the first of a new antibiotic class called pleuromutilins, available as a 1% ointment. This agent inhibits protein synthesis by binding to the 50S subunit on the ribosome. It is indicated for impetigo caused by S aureus or Streptococcus pyogenes.
Retapamulin ointment is in a new class of topical antimicrobials for treatment of localized impetigo caused by S pyogenes and methicillin-susceptible S aureus in children older than 9 months. It is applied twice daily for 5 days. It is not for mucosal use. Retapamulin has an excellent spectrum of activity, surpassing the bacterial spectrum of mupirocin.It has been shown to preserve its activity against bacteria that were resistant to multiple antibiotic drugs, such as methicillin, erythromycin, fusidic acid, mupirocin, azithromycin, and levofloxacin.
The spectrum of retapamulin also includes erythromycin-resistant S pyogenes, fusidic acid–resistant and mupirocin-resistant S aureus, and MRSA (including P-VL–positive strains). Retapamulin has been demonstrated to be as effective as topical fusidic acid and oral cephalexin, with a low rate of adverse events.In another study, retapamulin 1% ointment showed more efficacy than fusidic acid 2% ointment for the treatment of impetigo.
-
Topical sodium fusidate (fusidic acid).
Other topical antibiotics have been reported to have some benefit for the treatment of impetigo:
-
Clindamycin, useful in several MRSA infections.
-
Gentamycin ointment or cream
-
Hydrogen peroxide 1% cream, applied 2-3 times a day on the affected area for a maximum of 3 weeks.
-
Tetracycline. It is not widely prescribed because of the potential risk of skin photosensitivity reactions and because it is contraindicated in children younger than 8 years.
Drugs as sulfanilamide, nitrofurazone, silver sulfadiazine, because of their antibacterial spectrum, may also be considered for the treatment not only of impetigo but also for other community-acquired infections.
Systemic antibiotic therapy
When infections are widespread, complicated, or are associated with systemic manifestations (such as fever), are usually treated with systemic antibiotics that have gram-positive bacterial coverage. This therapy is also recommended if multiple incidents of pyoderma occur within daycare, family, or athletic team settings. Beta-lactamase resistant antibiotics (eg, cephalosporins, amoxicillin-clavulanate, cloxacillin, dicloxacillin) are recommended.
Cephalexin appears to be the drug of choice for oral antimicrobial therapy in children. Methicillin-resistant Staphylococcus aureus (MRSA) should be suspected in lesions that do not resolve with traditional antimicrobial therapy, in which case alternative antibiotics should be considered. These include trimethoprim-sulfamethoxazole, tetracycline, clindamycin, fluoroquinolones, linezolid and vancomycin. In areas with a high percentage of community-acquired MRSA, the empiric antibiotic choice depends on the prevalence and sensitivities of MRSA of the particular geographic region and should provide coverage for this possibility.
Erythomycin and clindamycin are alternatives in patients with penicillin hypersensitivity. Beside treatment, children with impetigo should avoid close contact with other children and should be excluded from school or day care for 24 hours after the initiation of antibiotics. Household members should be inspected for impetiginous lesions. In case of neonatal impetigo, should be evaluated not only household members but also the hospital nursery staff for pyodermas or asymptomatic bacterial carrier states.
Failure to treat other infected persons may result in continued transmission. Patients with atopic dermatitis should treat it, because this disease can predispose to skin infection. Treat the underlying disease has also been shown to decrease the pathogen count on the skin. Most important is a good personal hygiene:
-
nails short
-
hands cleaned and washed frequently with antibacterial soap and water or waterless antibacterial cleansers.
Patients with recurrent impetigo, asymptomatic family members and S Aureus carriers, should apply inside nostrils 2% mupirocin cream 2 times a day for 5 days each month to reduce colonization in the nose, axillae, umbilicus, genitals, peri-anal area.
